Assessment of Noninvasive Low-Frequency Ultrasound as a Means of Treating Injuries to Suspensory Ligaments in Horses: A Research Paper. Ugo Carrozzo a, *, Matteo Toniato a, Adrian Harrison b ABSTRACT Therapeutic ultrasound is a noninvasive technique, which is well tolerated by horses, does not need sedation, and can easily be performed in a routine clinical setting. Twenty-three client-owned sport horses were recruited at Clinica Equina San Biagio and included in this case study. Treatment of the injured suspensory ligament apparatus was administered using an EQ Pro, low-frequency therapeutic unit (38 kHz). The noninvasive treatment consisted of massaging the injured area in combination with a traditional ultrasound gel while maintaining the head of the device in direct contact with the injured area. The results indicate that 20 of the 23 horses in this study benefitted from EQ Pro treatment and, following a routine rehabilitation program, returned to competition status: a success rate of 87%. Furthermore, treatment duration was 3.3 ± 0.4 weeks on average, with a healthy outcome as assessed by ultrasound at 6.8 ± 1.9 weeks. Among the 23 horses in this study, 65% of them benefitted from EQ Pro treatment of a duration of just 3.3 weeks. It is concluded that EQ Pro therapy is a promising and effective form of treatment for horses with suspensory ligament injury. It is furthermore rapid and easy to use in the Equine Veterinary Clinic setting and does not require sedation. Future studies should now focus on the mechanisms by which this new treatment activates the healing process of the suspensory ligaments of injured horses. 1. Introduction Lameness remains the main cause of loss of performance in the equine industry, and suspensory ligament (SL) issues are one of the most common causes of lameness in horses [1e4]. Injury to the main SLs or to either one or even both of the SL branches is common in all types of athletic horses [5,6]. Indeed, injury to flexor tendons or the SLs has been reported to account for up to 46% of all limb injuries in performance and racehorses [1,2,7]. Moreover, it has been stated that “lameness often has been slow to resolve (2e3 months) with a total duration of convalescence ranging from 3 to 6 months” [8]. 2. Materials and Methods 2.1. Ethics There were no ethical issues associated with this study. The equipment used complied with both Conformite Europeenne and Federal Communications Commission regulations and was noninvasive in its nature. The study was carried out according to the guidelines laid out in the Helsinki Declaration (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/), and care was taken not to unduly stress the horses. The data collected were from privately owned horses over a period of 24 months (January 2016 to December 2017), and as such, this work should be seen as a retrospective study. 2.2. Animals Animals were recruited through the Clinica Equina San Biagio. The following criteria were adopted 2.3. Study The injuries outlined in this study all occurred naturally, which adds a degree of difference in terms of their entity and their severity. The horse population also differed greatly in terms of age and breed. This study was also subject to logistics issues in terms of treatment between individual horses (three treatment protocols were adopted: Protocol 1: acute cases; Protocol 2: subacute cases; Protocol 3: chronic cases: for details see below), with some horses skipping some treatment sessions or indeed finishing therapy earlier than had initially been planned. All these variables should be taken into account when considering the noticeably different outcomes of this study with regard to the time spent to heal lesions as well as the time spent before a return to regular sport activities. 2.4. Assesment Each horse was subjected to a complete physical and lameness evaluation. Any palpable abnormalities (thickening, swelling, and so forth) were recorded. The horses were initially evaluated by an experienced lameness clinician (U.C. and M.T.) while moving at a walk and a trot in a straight line and in a circle, both on a hard and a soft surface. 2.5. Treatment The device used for low-frequency US therapeutic treatments was an EQ Pro (EQ Veterinary, Daytona Beach, Florida; www.eqveterinary.com). This device produces a low-frequency therapeutic US, which operates at 38 kHz (±2 kHz). The unit comes with six different color-coded transducers, featuring continuous and pulsed emission and with different transducer shapes to allow treatment of different musculoskeletal pathologies in different areas of the horse’s body: - Red transducer (flat head – dcontinuous emission) generates acoustic effects and thermal effects. The red transducer is ideal to treat large and flat areas and when heat is a desired effect. All transducers induce acoustic effects within targeted tissues. Continuous emission transducers (red and yellow transducers) are mainly indicated for chronic conditions and acute muscle conditions or generally speaking when there is the need to increase vascularization. Continuous emission transducers can also be used to soften scar tissue. 2.6. Rehabilitation The rehabilitation plan adopted throughout this study was based on the following protocol. For the first 4 weeks after diagnosis, exercise was by walking only (10 minutes twice daily). From Weeks 4 to 8 after diagnosis, a gradual and increasing exercise plan of both walking and trotting was adopted up to a period of 20 minutes. This was also augmented by a gradually increasing period of canter exercise (1 minute initially and thereafter an additional minute every third day). This rehabilitation plan was carefully adjusted for each horse depending on the severity of their injury as well as both clinical and ultrasonographic examinations. 3. Results 3.1. General Findings The summary of the details and diagnosis of the 23 horses included in this study can be found in Table 1. 3.2. Protocol 1 – Acute Case During the first week after diagnosis, EQ Pro treatment was provided 6 days a week using the blue transducer (flat transducer with pulsed emission) at 70% to 80% of full power for a period of 10 to 15 minutes, either once or twice a day (depending on the severity of the tissues’ edema). During the second week after diagnosis, EQ Pro treatment was provided 6 days a week using the blue transducer (flat transducer with pulsed emission) at 80% to 95 % of full power for a period of 6 minutes. Thereafter, the gray transducer (focused convex transducer with pulsed emission) was used at 80% to 95% of full power for a period of 5 minutes. 3.3. Protocol 2 – Subacute Case During the first week after diagnosis, EQ Pro treatment was provided 6 days a week using the blue transducer (flat transducer with pulsed emission) at 70% to 80% of full power for a period of 10 to 15 minutes. 3.4. Protocol 3 – Chronic Case During the first week after diagnosis, EQ Pro treatment was provided 6 days a week using the red transducer (flat transducer with continuous emission) at 70% to 85% of full power for a period of 6 minutes. Thereafter, the gray transducer (focused convex transducer with pulsed emission) was applied at 80% to 95% of full power for a period of 5 minutes. 4. Exemplary Case 4.1. Subject 1 with Clinical History Case number 1 was a 16-year-old Polish warmblood gelding used for middle-level dressage competitions. The horse showed left hindlimb acute 3/5 lameness the day after hard work and an obvious swelling of the distal plantar aspect of the left metatarsal region. A diagnostic US examination revealed not only a conspicuous bilateral lesion of the SL branches, but an increase in their CSA, hypoechogenic confluent areas, and moderate periligamentous soft tissue swelling, as shown in Fig. 3A. 5. Summary of Observations This study has shown that an EQ treatment duration of 3.3 ± 0.4 weeks on average (minimum 3.0, maximum 4.0, and median 3.0) gave rise to a healthy outcome, as assessed by US after a period of 6.8 ± 1.9 weeks (minimum 4.0, maximum 10.0, and median 8.0). The follow-up diagnostic US images for the horses included in this study showed a clear benefit of EQ Pro treatment with reduced swelling, and as a consequence, a reduction in the CSA of the injured region (see Table 3). Indeed, there was a significant 15%decrease (P < .0001) in the CSA of the SLs from 2.71 cm at the time of diagnosis to 2.30 cm at the time of follow-up. Moreover, there was found to be an improvement in echogenicity, an indication that cellular repair had been initiated. In terms of fiber alignment, 39% of the horses presented with a fiber alignment of <50% at the time of diagnosis, but by end of the study at follow-up, only 4% of the horses had a fiber alignment of <50%, and 78% had a fiber alignment of 100%. 6. Discussion To the best of the authors knowledge, this is the first study, albeit a retrospective one, that documents the effects of low-frequency US on the suspensory system of horses, reporting (1) the duration of treatment in relation to the severity of the injury sustained, (2) the point in time at which the injury was deemed to be healthy using US, and (3) the success with regard to return to full competition status. 7. Conclusion This study describes a physiotherapy treatment approach based on the use of low-frequency US, in connection with a rehabilitation program that is tailored to each single case, and one that takes into account the age, discipline, type of lesion, previous treatment, and response to treatment. It is shown that EQ Pro treatment requires on average a period of 3.3 ± 0.4 weeks to result in a successful outcome (return to full competition status) for 87% of the 23 horses included in this study. It is furthermore quick and easy to use in the Equine Veterinary Clinic setting and does not require sedation. There is now a need for more detailed studies into the mechanisms by which low-frequency US is able to activate autophagy, as well as the role of autophagy in the healing process of the SLs of injured horses. CRediT authorship contribution statement Ugo Carrozzo: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing -review & editing. Matteo Toniato: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – review & editing. Adrian Harrison: Formal analysis, Project administration, Software, Visualization, Writing -original draft, Writing – review & editing. Acknowledgments The authors are indebted to the horse owners for allowing them to perform these noninvasive treatments and measurements. References [1] Kasashima Y, Takahashi T, Smith RKW, Goodship E, Kuwano A, Ueno T, Hirano S. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet J 2004;36:346-50. [2] Williams RB, Harkins LS, Hammond CJ, Wood JLN. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet J 2001;33: 478-86. [3] Murray RC, Dyson SJ, Tranquille C, Adams V. Association of type of sport and performance level with anatomical site of orthopaedic injury diagnosis. Equine Vet J 2006;38:411-6. [4] Hill AE, Gardner IA, Carpenter TE, Lee CM, Hitchens PL, Stover SM. Prevalence, location and symmetry of noncatastrophic ligamentous suspensory apparatus lesions in California Thoroughbred racehorses, and association of these lesions with catastrophic injuries. Equine Vet J 2016;48:27-32. [5] Dyson S. The lowdown on high suspensory disease (proximal suspensory desmitis). AAEP; 2016. https://aaep.org/horsehealth/lowdown-high-suspensory-disease-proximal-suspensory-desmitis. [6] Ferraro GL, Stover SM, Whitcomb MB. Suspensory ligament injuries in horses. Davis: Center for Equine Health, School of Veterinary Medicine, University of California; 2007. http://www.vetmed.ucdavis.edu/ceh/local_resources/pdfs/Pubs-SuspBrochure-bkm-sec.pdf. [7] Dahlgren LA. Pathobiology of tendon and ligament injuries. Clin Tech Equine Pract 2007;6:168-73. [8] Dyson SJ, Arthur RM, Palmer SE, Richardson D. Suspensory ligament desmitis. Vet Clin North Am Equine Pract 1995;11:177-215. [9] Kovac M, Litvin YA, Aliev RO, Zakirova EY, Rutland CS, Kiyasov AP, Rizvanov AA. Gene therapy using plasmid DNA encoding VEGF164 and FGF2 genes: a novel treatment of naturally occurring tendinitis and desmitis in horses. Front Pharmacol 2018;9:978. [10] Docheva D, Muller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev 2015;84:222-39. [11] Martinek V, Huard J, Fu FH. Gene therapy in tendon ailments. In: Maffulli N, Renstr€om P, Leadbetter WB, editors. Tendon injuries. London: Springer; 2005. [12] Waselau M, Sutter WW, Genovese RL, Bertone AL. Intralesional injection of platelet-rich plasma followed by controlled exercise for treatment of midbody suspensory ligament desmitis in standardbred racehorses. J Am Vet Med Assoc 2008;232:1515-20. [13] Russell JW, Russell TM, Vasey JR, Hall MS. Autologous bone marrow aspirate for treatment of superficial digital flexor tendonitis in 105 racehorses. Vet Rec 2016;179:69. [14] Seabaugh KA, Thoresen M, Giguere S. Extracorporeal shockwave therapy increases growth factor release from equine platelet-rich plasma in vitro. Front Vet Sci 2017;4:205. [15] Romagnoli N, Rinnovati R, Ricciardi G, Lambertini C, Spinella G, Spadari A. Clinical evaluation of intralesional injection of platelet-rich plasma for the treatment of proximal suspensory ligament desmitis in horses. J Equine Vet Sci 2015;35:141-6. [16] Vandenberghe A, Broeckx SY, Beerts C, Seys B, Zimmerman M, Verweire I, Suls M, Spaas JH. Tenogenically induced allogenic mesenchymal stem cells for the treatment of proximal suspensory ligament desmitis in a horse. Front Vet Sci 2015;2:1-7. [17] Juneja SC, Schwarz EM, OKeefe RJ, Awad HA. Cellular and molecular factors in flexors in flexor tendon repair and adhesion: a histological and gene expression analysis. Connect Tissue Res 2013;54:218-26. [18] Sodersten F, Hultenby K, Heinegard D, Johnstonn C, Ekman S. Immunolocalization of collagens (I and III) and cartilage oligomeric matrix protein in the normal and injured equine superficial digital flexor tendon. Connect Tissue Res 2013;54:62-9. [19] Sharma P, Maffulli N. Biology of tendon injury: healing, modelling and remodelling. J Musculoskelet Neuronal Interact 2006;6:181-90. [20] Yang C, Rothrauff B, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and development paradigm. Birth Defects Res C Embryo Today 2013;99:203-22. [21] Shi A, Xu Z, Lundt J, Tamaddoni HA, Worlikar T, Hall TL. Integrated histotripsy and bubble coalescence transducer for rapid tissue ablation. IEEE Trans Ultrason Ferroelectr Freq Control 2018;65:1822-31. [22] Wu Y, Liu X, Qin Z, Hu L, Wang X. Low-frequency ultrasound enhances chemotherapy sensitivity and induces autophagy in PTX-resistant PC-3 cells via the endoplasmic reticulum stress-mediated P13K/Akt/mTOT signaling pathway. Onco Targets Ther 2018;11:5621-30. [23] Mohamed E, Cao Y, Rodriguez PC. Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy. Cancer Immunol Immunother 2017;66:1069-78. [24] Filippi-Chiela EC, Viegas MS, Thome MP, Buffon A, Wink MR, Lenz G. Modulation of autophagy by calcium signalosome in human disease. Mol Pharmacol 2016;90:371-84. [25] Li Y, Zhang J, Liu T, Chen Y, Zeng X, Chen X, He W. Molecular machinery of autophagy and its implication in cancer. Am J Med Sci 2012;343: 155-61. [26] Bhat P, Kriel J, Shubha P, Basappa B, Shivananju NS, Loos B. Modulating autophagy in cancer therapy: advancements and challenges for cancer cell death sensitization. Biochem Pharmacol 2018;147:170-82. [27] Zeng C, Liu J, Wang H, Zhou Y, Wu J, Yan G. Correlation between autophagy and collagen deposition in patients with pelvic organ prolapse. Female Pelvic Med Reconstr Surg 2018;24:213-21. [28] Ryter SW, Cloonan SM, Choi AM. Autophagy: a critical regulator of cellular metabolism and homeostasis. Mol Cells 2013;36:7-16. [29] Dijkmans PA, Juffermans LJ, Musters RJ, van Wamel A, ten Cate FJ, van Gilst W, Visser CA, de Jong N, Kamp O. Microbubbles and ultrasound: from diagnosis to therapy. Eur J Echocardiogr 2004;5:245-56. [30] Shindo Y, Kato K, Ichishima Y, Iseki Y, Tokutake R, Ikuta F, Takahashi K. Evaluation of deep thermal rehabilitation system using resonant cavity applicator during knee experiments. Conf Proc IEEE Eng Med Biol Soc 2018;2018:3220-3. [31] Collis J, Manasseh R, Liovic P, Tho P, Ooi A, Petkovic-Duran K, Zhu Y. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics 2010;50:273-9. [32] Gul EE, Abuelatta R, Haseeb S, Melhem M, Al Amoudi O. Venoplasty of a chronic venous occlusion with “diathermy” for cardiac device lead placement. Indian Pacing Electrophysiol J 2018;19:27-9. [33] Bhatnagar S, Schiffter H, Coussios CC. Exploitation of acoustic cavitation-induced microstreaming to enhance molecular transport. J Pharm Sci 2014;103:1903-12. [34] El Ghamrawy A, de Comtes F, Koruk H, Mohammed A, Jones JR, Choi JJ. Acoustic streaming in a soft tissue microenvironment. Ultrasound Med Biol 2018:208-17. [35] Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique e no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand 2000;71:475-9. [36] Cook J. In search of the tendon holy grail: predictable clinical outcomes. Br J Sports Med 2009;43:235. [37] Olesen JL, Heinemeier KM, Langberg H, Magnusson SP, Kjaer M, Flyvbjerg A. Expression, content, and localization of insulin-like growth factor I in human achilles tendon. Connect Tissue Res 2006;47:200-6. [38] Holm L, Van Hall G, Rose AJ, Miller BF, Doessing S, Richter EA, Kjaer M. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab 2009;298:E257-69. [39] Langberg H, Ellingsgaard H, Madsen T, Jansson J, Magnusson SP, Aagaard P, Kjaer M. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand J Med Sci Sports 2007;17:61-6. [40] Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross- linking of the patellar tendon in old and young men. J Appl Physiol 2009;107:880-6. [41] Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec 2010;3:29-32. [42] Jarvinen TA, Kannus P, Paavola M, Jarvinen TL, Jozsa L, Ja€rvinen M. Achilles tendon injuries. Curr Opin Rheumatol 2001;13:150-5. [43] Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheumatol 2007;56: 871-81. [44] Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse model. J Shoulder Elbow Surg 2005;14:79S-83S. [45] Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson 3rd JD, Carpenter JE. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng 2002;30:1057-63. [46] Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 1996;5:383-92. [47] Glazebrook MA, Wright JR, Langman M, Stanish WD, Lee JM. Histological analysis of Achilles tendons in an overuse rat model. J Orthop Res 2008;26: 840-6.
Journal of Equine Veterinary Science 80 (2019) 80-89
a) Clinica Equina San Biagio, San Biagio di Argenta, FE, Italy
b) Department of Pathobiological Sciences, Faculty of Health & Medical Science, Copenhagen University, Frederiksberg C, Copenhagen, Denmark
The main function of the SL is to prevent overextension of the fetlock joints during the weight-bearing phase of a horse’s stride. Injuries to the SL typically arise when the load placed on them exceeds their combined strength. For the equine SL, this most often occurs at the middle of the stance phase of the stride [6].
Following an injury to a ligament, the healing process follows a very predictable sequence of inflammation, proliferation or repair, and remodeling [9]. However, an underlying problem in terms of healing is the fact that ligaments are relatively poorly vascularized, and a good vascular system is required to facilitate cell infiltration and the supply of growth factors needed for full tissue healing [10].
It is for this reason that the use of recombinant proteins and gene therapy has been looked at in terms of a promising approach to treatment of such injuries, based on its success in muscle disorders in humans [11]. Indeed, as an approach, it has been shown to be very promising in horses presenting with injuries to the superficial digital flexor tendon [9]. Other forms of treatment include platelet-rich plasma (PRP) [12], autologous bone marrow aspirate [13], and extracorporeal shockwave therapy [14]. Indeed, a combination of both PRP and extracorporeal shockwave has recently been proposed as a means of increasing the expression of important growth factors in regions of soft tissue injury, thereby creating a synergistic effect and hastening the healing process [14].
The use of low-frequency therapeutic ultrasound (US) to treat equine conditions is as yet a new and evolving science. As a technique, therapeutic US is noninvasive and well tolerated by horses without the need of sedation. Furthermore, it can be easily performed in a routine clinical setting. EQ Pro low-frequency therapeutic US generates intense acoustic effects, thermal effects, and mechanical vibration. The acoustic effects are cavitation, acoustical streaming, and microstreaming. Thermal effects are induced by EQ Pro continuous emission transducers, heating deep tissues with a minimal increase in temperature of the superficial tissues. Mechanical vibration is induced by EQ Pro pulsed emission transducers: the intermittence of on-off cycles allows heat dissipation by blood circulation and creates a therapeutic mechanical vibration.
The aim of this case study has therefore been to examine the efficacy of low-frequency US and present the response to treatment in combination with a careful exercise program in 23 horses.
Inclusion criteria are as follows: (1) unilateral lameness; (2) swollen or thickened SL or its branch(es); (3) positive diagnostic analgesia; and (4) ultrasonographic evidence of acute or chronic, focal or diffuse simple or complicated lesion(s) of SLs.
Exclusion criteria are as follows: (1) lameness complicated by multiple injuries that involved other structures, (2) horses that had developed drop off of the fetlock with consequent elongation of the SL, and (3) dystrophic mineralization of the SL and/or of the periligamentous fibrous tissue.
Twenty-three horses met both the inclusion and exclusion criteria. The population consisted of 14 geldings, 7 mares, and 2 stallions, with a breakdown as follows: one polish-warmblood, three Hanoverians, six Holsteiners, three KWPN Royal Dutch Sports horses, one Oldenburg, four Sella Italiano, two English Thoroughbreds, one Belgian Warmblood, one Selle Français, and one Quarter Horse. The average (mean ± standard deviation) weight and age were 584 ± 54 kg and 11 ± 3 years, respectively.
Diagnostic analgesia was performed to confirm the source of pain. Horses with lesion of suspensory branch were positive (>80% improvement of lameness) to low 4-point block (3 cc mepivacaine 2% around palmar/plantar metacarpal/tarsal nerves and 4 cc around palmar/plantar nerves).
Horses with lesion of the origin of SL were blocked with small amount of anesthetic solution (3 cc mepivacaine 2%) directly injected into the lesion or around tibial nerve (one horse) with US-guided injection.
Thereafter, the SLs were examined using a diagnostic US unit (Esaote Alpha diagnostic unit; Esaote SpA, Genoa, Italy; performed by U.C. and M.T.) not only to locate the site of lameness but also to assess the degree of the injury (for details see Table 1 and Fig. 1). Clinical relevance of lesion(s) was evaluated and confirmed by ul-trasonographic examination usually complemented by a radio-graphic examination to highlight osseous or joint abnormalities. There was not a strict and consistent correlation between the lameness score and the severity of lesions as evaluated by the US images. An overall evaluation of the severity of an individual injury was therefore taken using a combination of clinical records, diagnostic imaging, and patient indications.
Transverse and longitudinal sections of proximal, body, and branches of SL were taken, and most representative images of the lesion(s) were digitized using commercially available DICOM viewer and analyzer software (Osirix MD, PIXMEO Sarl 2016); cross-sectional areas (CSAs) were measured by manually tracing the borders of the region of interest and automatically calculating the area included; longitudinal sections were analyzed for fiber alignment percentage and classified as poor (<50% fibers aligned), fair (=50% fibers aligned) and good (>50% fibers aligned). When available, follow-up images were taken and analyzed as well, following the same criteria.
An appropriate treatment plan for rehabilitation and recovery was built on an accurate diagnosis and detailed understanding of the condition to be treated. Treatment areas were defined after ultrasonography of the injured area (Esaote Alpha diagnostic unit; Esaote SpA, Genoa, Italy). Finally, the safety of the practitioner, horse, and equipment were considered at all times because equine patients were prepared for low-frequency US treatment in such a way to prevent uncontrolled kicking, distress, or pain (horses were simply cross-tied to prevent them from turning around). The stress and/or pain of each subject was estimated during the treatment procedures, where such traits as (1) tendency to move forward or backward and or side by side more than four times per minute, (2) repeated, insisted pawing, and (3) sudden, hasty limb subtraction were relied on.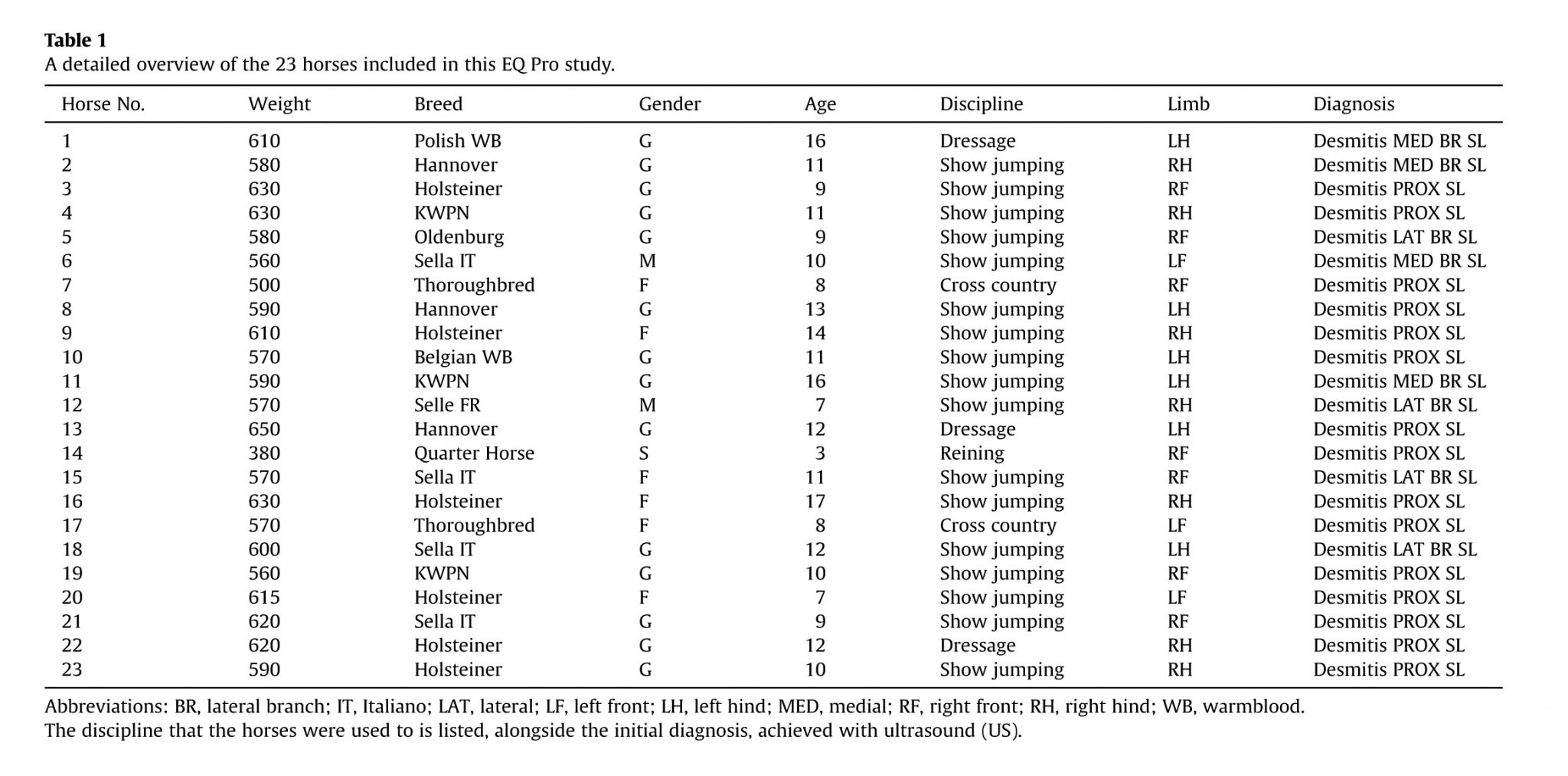
– Yellow transducer (concave head – continuous emission) generates acoustic effects and thermal effects. The yellow transducer is ideal to treat small and rounded areas and when heat is a desired effect.
– Blue transducer (flat head – pulsed emission) generates acoustic effects and mechanical vibration. The blue transducer is ideal to treat large and flat areas and when heat is an undesired effect.
– Green transducer (concave head – pulsed emission) generates acoustic effects and mechanical vibration. The green transducer is ideal to treat small and rounded areas and when heat is an undesired effect.
– Gray transducer (focused convex head – pulsed emission) generates acoustic effects and mechanical vibration. Because of the small contact area, US emission is focused. The focused convex head has a divergent emission.
– White transducer (focused concave head – pulsed emission) generates acoustic effects and mechanical vibration. Because of the small contact area, US emission is focused. The focused concave head has a convergent emission.
Pulsed emission transducers (blue and green transducers) are mainly indicated for acute or subacute conditions or generally speaking when the user does not want to add heat to an existing inflammation because heat is mostly dissipated by blood circulation. Pulsed emission transducers can also be used to drain edema and hematoma.
The focused transducers (gray and white transducers) can be used in acute, subacute, or chronic conditions when very intense acoustic effects are desired.
EQ Pro allows the user to set the intensity of the power output and treatment duration. Increasing the intensity of the power output and the treatment duration allows the user to generate more intense effects and also to reach deeper targets. The two advantages of using low-frequency therapeutic US versus high-frequency US are increased intensity of acoustic effects and depth of penetration.
EQ Pro therapy does not require treatment areas to be shaved, only that they are cleaned with water and left wet so as to facilitate the EQ Pro transduction. US-specific gel was applied to both the contact surface of the EQ Pro transducer and directly to the treatment area. This was done so as to further facilitate EQ Pro penetration but also to assist the transducer head to smoothly glide over the treatment area. Subsequent applications of US gel were made when it was felt that insufficient gel was present at the treatment site.
Treatment sessions commenced with the EQ Pro unit set to its 50% power output setting so that horses could become accustomed to the EQ Pro therapy. After a minute of treatment at this setting, the power output was then raised to the desired level. Treatment was performed by massaging the selected area of the horse while maintaining the US transducer in direct contact with horse’s gel enriched hair. It can be seen in Fig. 2 that the transducer was applied to the region of the horse to be treated using slight pressure, and that massage was applied in both circular and linear directions.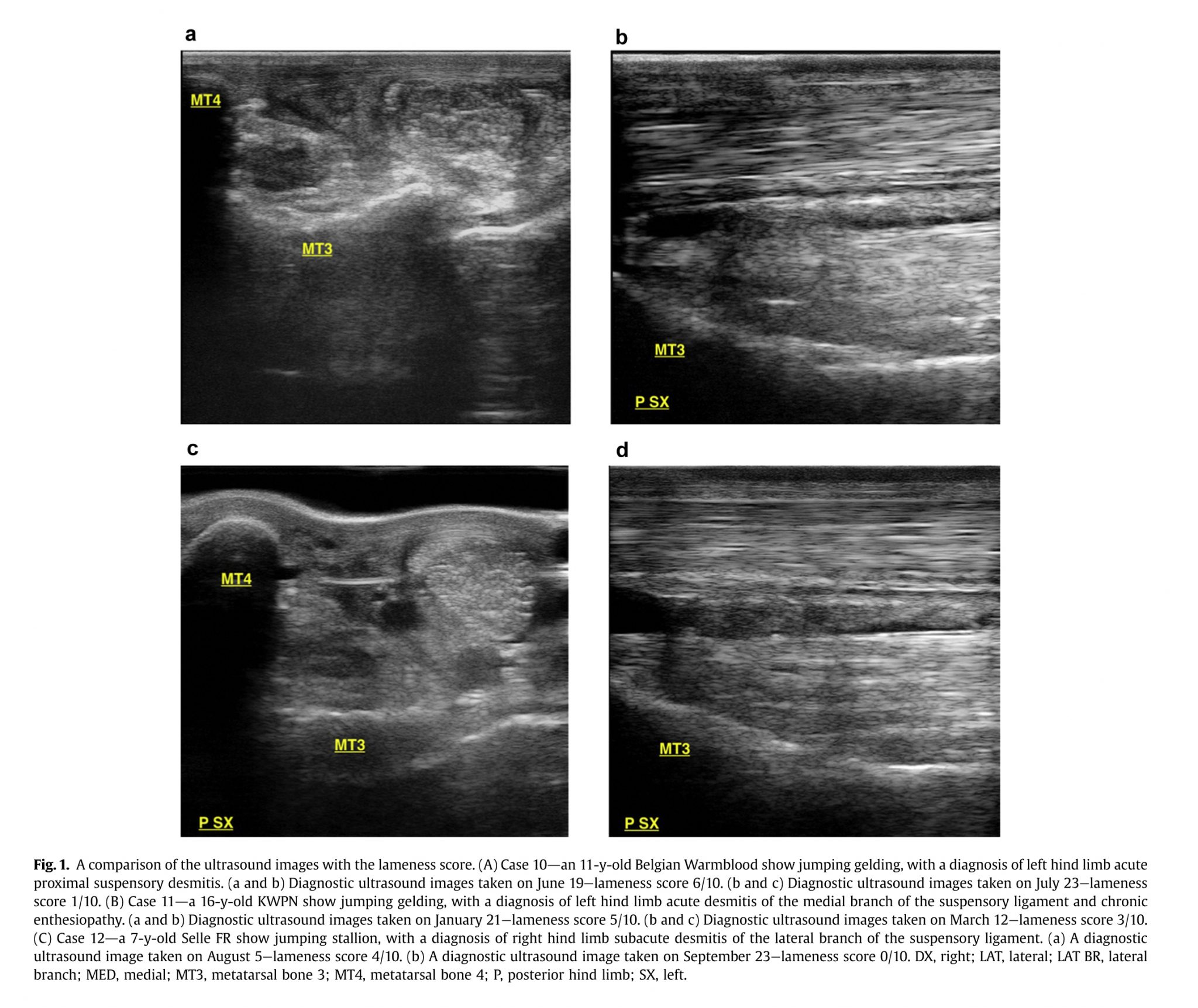
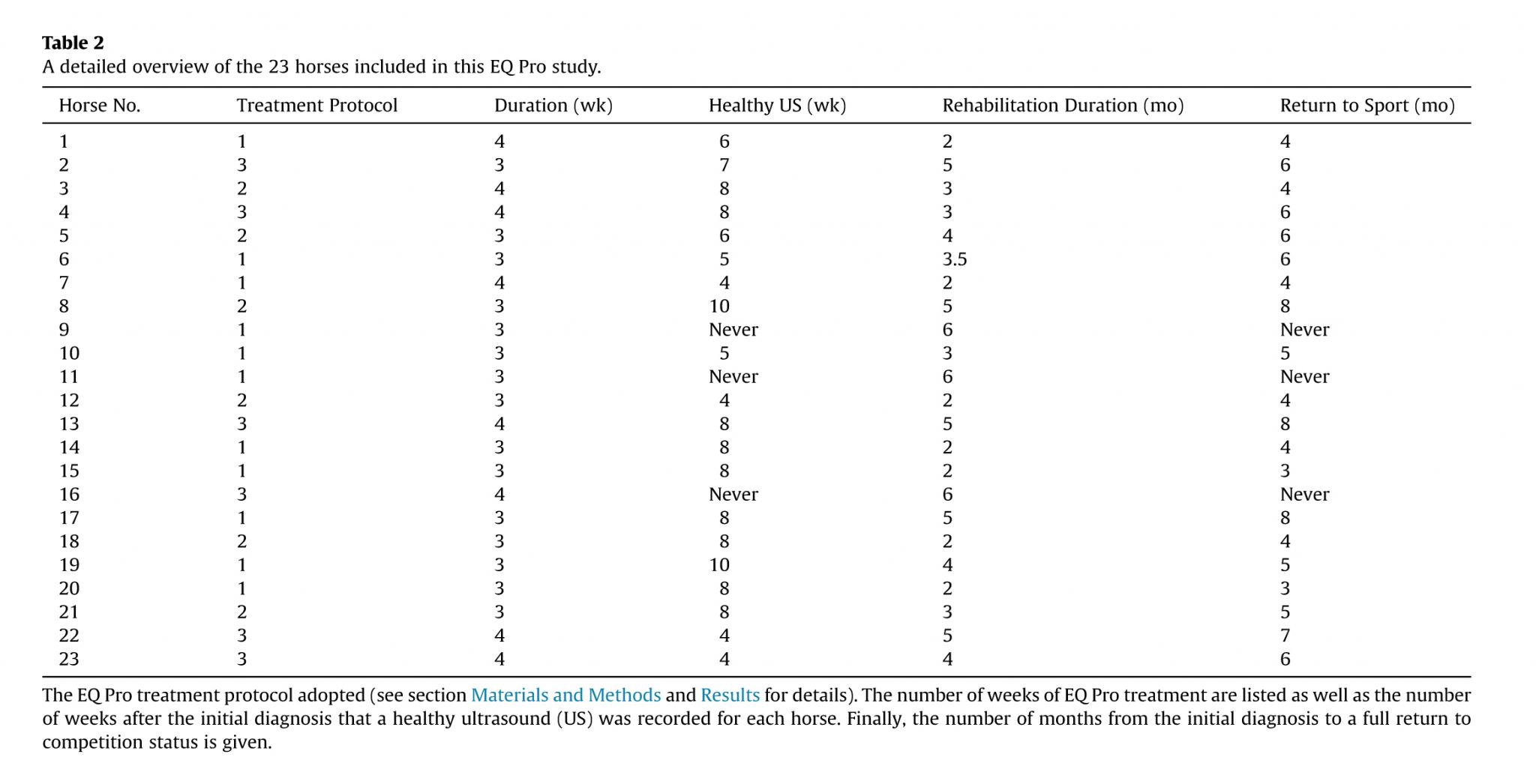
It was found that of 23 horses included in this study, 56% had an injury to their hind-limb, and in 65% of the cases, this was to the origin of the SL. When the suspensory branches were affected (n = 8), the injury was divided evenly between the medial and the lateral branch.
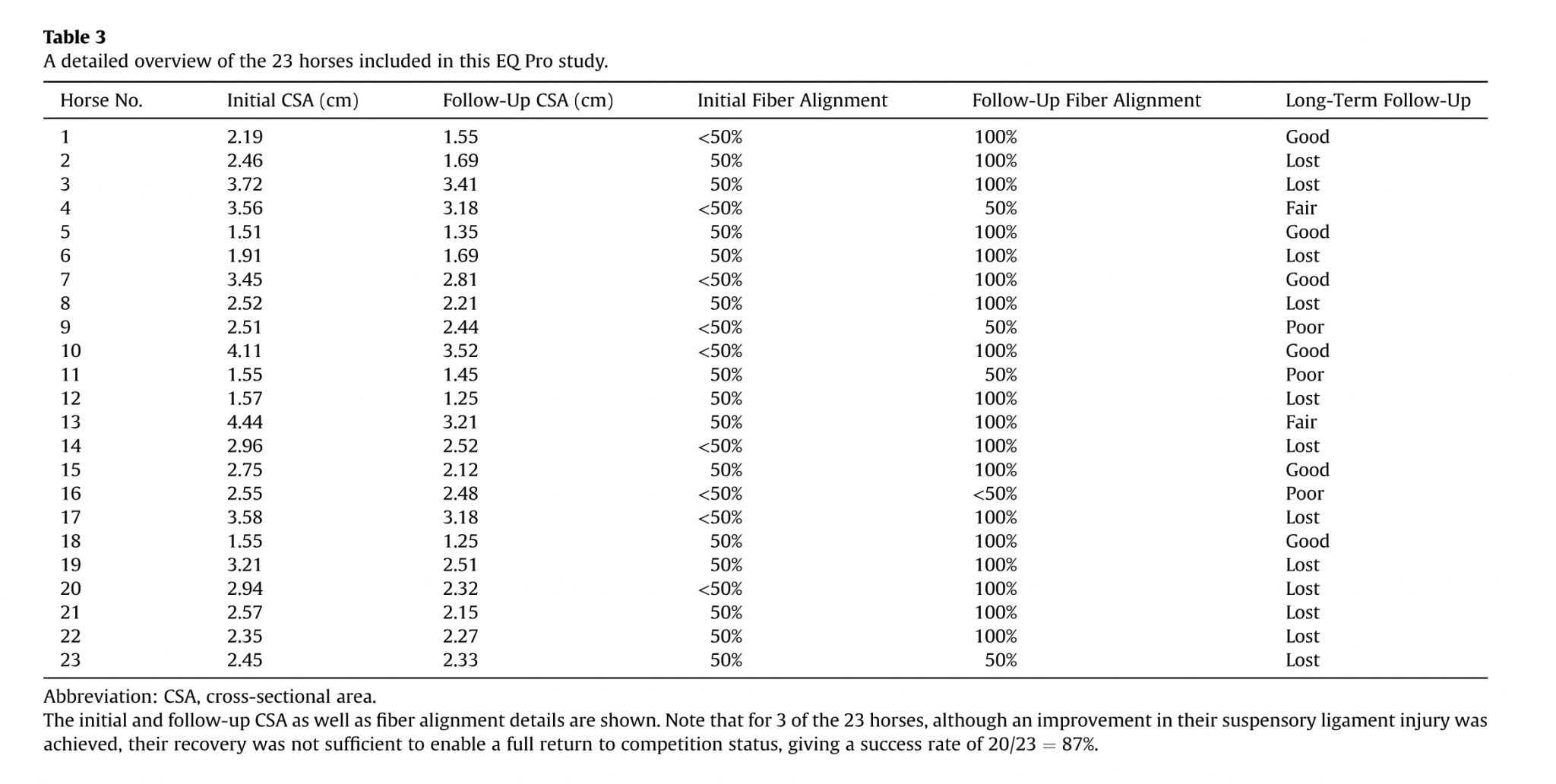
Evaluation of success was defined by clinical and US examination to assess resolution of lameness and US improvement. The outcome of the EQ Pro treatment regimen was found to have a success rate of 87% as 20 of the 23 horses returned to full competition status after a period of recovery or rehabilitation training. Although a clear improvement was found with EQ Pro treatment for the three remaining horses, they did not improve enough to return to their chosen sports activity (see Table 2).
Three different treatment protocols were used in this study, depending on whether the injury was assessed as being acute, subacute, or chronic. Treatment protocols have been derived from human physiotherapy protocols and modified accordingly with the experience of the authors. All the transducers were applied while (1) taking care to keep the treatment site covered with gel and (2) moving the transducer head throughout the treatment.
During the third week, and when deemed necessary based on ultrasonographic and clinical examinations during the fourth week after diagnosis, EQ Pro treatment was provided three times a week using the red transducer (flat transducer with continuous emis-sion) at 70% to 85% of full power for a period of 6 minutes. There-after, the blue transducer (flat transducer with pulsed emission) was applied at 80% to 95% of full power for a period of 6 minutes. Then finally the gray transducer (focused convex transducer with pulsed emission) was applied at 80% to 95% of full power for a period of 5 minutes.
During the second week after diagnosis, EQ Pro treatment was provided three times a week using the blue transducer (flat transducer with pulsed emission) at 80% to 95% of full power for a period of 6 minutes. Thereafter, the gray transducer (focused convex transducer with pulsed emission) was used at 80% to 95% of full power for a period of 5 minutes.
During the third week, and when deemed necessary based on ultrasonographic and clinical examinations during the fourth week after diagnosis, EQ Pro treatment was provided twice a week using the red transducer (flat transducer with continuous emission) at 70% to 85% of full power for a period of 6 minutes. Thereafter, the blue transducer (flat transducer with pulsed emission) was applied at 80% to 95% of full power for a period of 6 minutes. Then finally the gray transducer (focused convex transducer with pulsed emission) was applied at 80% to 95% of full power for a period of 5 minutes.
During the second week after diagnosis, EQ Pro treatment was provided three times a week using the red transducer (flat transducer with continuous emission) at 70% to 85 % of full power for a period of 6 minutes. Thereafter, the blue transducer (flat transducer with pulsed emission) was used at 80% to 95 % of full power for a period of 6 minutes. Then, finally the gray transducer (focused convex transducer with pulsed emission) was applied at 80% to 95%of full power for a period of 5 minutes.
During the third week and when deemed necessary based on ultrasonographic and clinical examinations during the fourth week after diagnosis, EQ Pro treatment was provided twice a week using the red transducer (flat transducer with continuous emission) at 70% to 85% of full power for a period of 6 minutes. Thereafter, the blue transducer (flat transducer with pulsed emission) was used at 80% to 95% of full power for a period of 6 minutes. Then finally the gray transducer (focused convex transducer with pulsed emission) was applied at 80% to 95% of full power for a period of 5 minutes.
The horse started with treatment protocol 1dacute case: for the first 6 days after diagnosis, EQ Pro treatment was given twice a day on a daily basis using the blue transducer (flat transducer with pulsed emission) at 80% power output for 10 minutes. During the second week EQ Pro treatment was given once a day, 6 days a week, using the blue transducer (flat transducer with pulsed emission) at 80% power output for 6 minutes, followed by the gray transducer (focused convex transducer with pulsed emission) at 80% power output for 5 minutes.
Then in the third and fourth weeks, EQ Pro treatment was given three times a week. Each treatment session comprised the use of the red transducer (flat transducer with continuous emission) at 70% power output for 6 minutes, followed by the blue transducer (flat transducer with pulsed emission) at 80% power output for 6 minutes, and finally the gray transducer (focused convex transducer with pulsed emission) was used at 80% power output for 5 minutes.
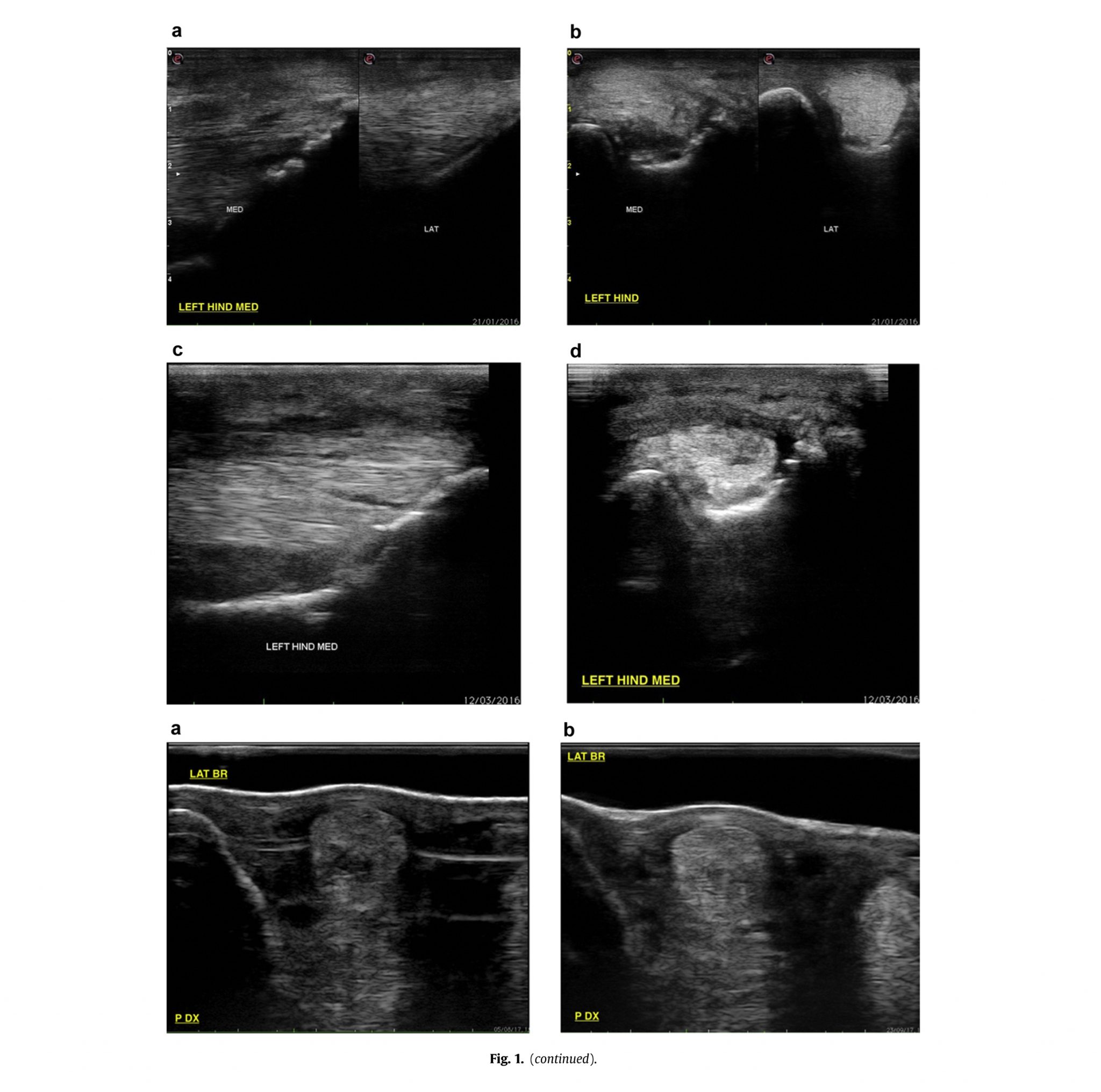
In the sixth week, a follow-up diagnostic US examination showed improved echogenicity of the SL branches, a reduction in their CSA and an almost complete reduction in the periligamentous thickening, as shown in Fig. 3B. Subsequently, the horse was managed with a rehabilitation program (see section Materials and Methods) for a period of 2 months, before resuming regular sport activity after 4 months, and reinjury was not reported.
For details of the other 22 horses and their treatment period and outcome, see Table 2.
Treatment with EQ Pro was followed up with a standard reha-bilitation program that, on average, lasted for 3.6 ± 1.4 months (minimum 2.0, maximum 6.0, and median 3.5), with horses returning to full competition status after a period of 5.3 ± 1.6 months on average (minimum 3.0, maximum 8.0, and median 5.0).
Although this study population is too small to enable statistically significant results, front limb proximal SLs and medial or lateral branch desmopathy showed a better rate of improvement in terms of time to return to sport in the opinion of the authors.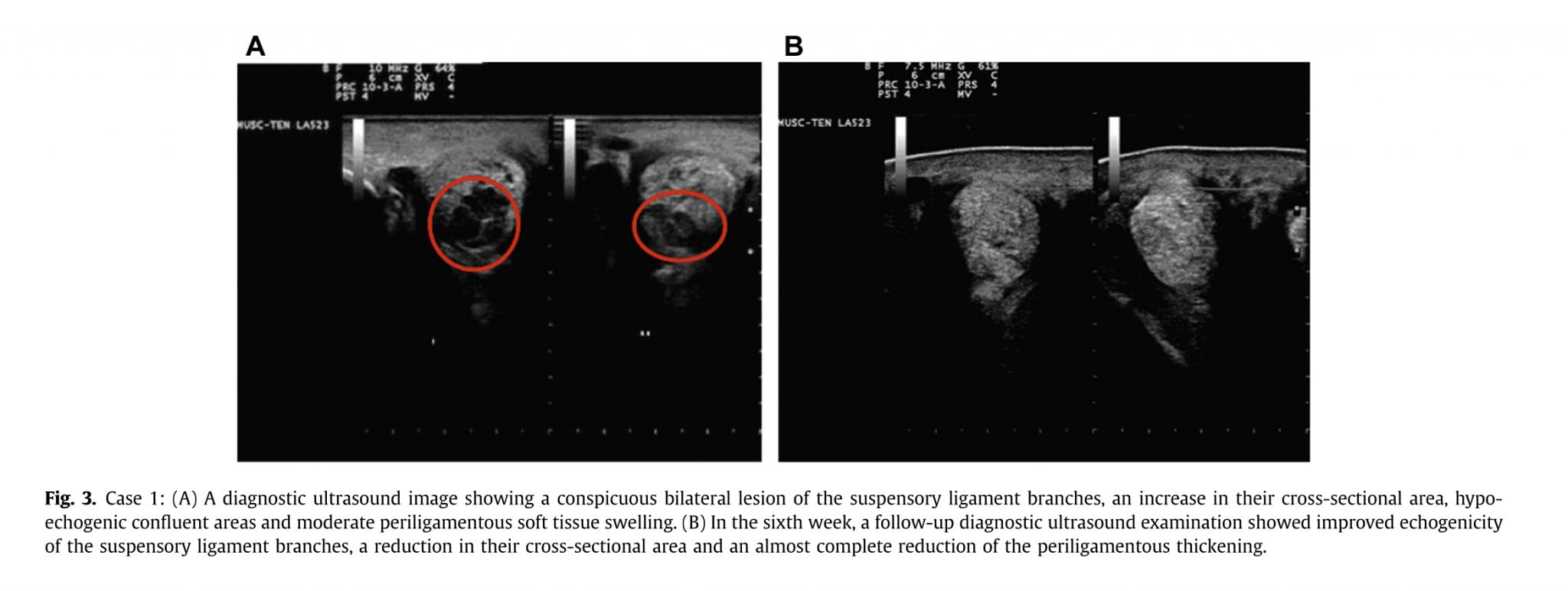
This study has shown that for the 23 horses examined, 20 were successfully returned to competition status, and this occurred on average within a time frame of 21 weeks. Moreover, 20 of the 23 horses were deemed healthy at an US examination after, on average, 6.8 weeks and were rehabilitated for, on average, 14 weeks. In a study involving PRP treatment, horses returned to competition status between 12 and 24 weeks after an acute proximal suspensory ligament desmitis [15]. However, PRP resulted in horses returning to exercise first after a period of 14.5 weeks on average [15], compared to the 6.8 weeks reported in the present study. Stem cell treatment has also been applied to proximal suspensory liga-ment desmitis, with a report of a return to preinjury performance status 32 weeks after the start of regenerative treatment [16], compared with the average time frame of 21 weeks in the present low-frequency US study.
One of the problems associated with repair of ligaments is that often rather more collagen III is laid down in the newly developing matrix [17]. Ligaments and tendons are arranged as long, thin collagenous fibers (collagen I) with an inherent characteristic waviness also known as crimp [6]. Collagen III fibers are rather smaller and less elastic than collagen I fibers [18]. During remodeling, the collagen fibers become aligned in the direction of the dominant stress, and the collagen III fibers are replaced with collagen I fibers [19]. However, in many cases, the repaired tissue remains biochemically and ultrastructurally abnormal, even up to a year after injury, and does not completely regain the biomechanical properties it possessed initially [20].
It has been stated that the degree of ultrasonographic abnormality usually reflects the severity of the lameness [5]. This study has been able to confirm this, as the US images and their severity matched with very close approximation, the degree of lameness noted for the 23 cases described. As it can be deduced from Table 3, CSA and fiber alignment patterns improved after treatment with the EQ Pro, and matched the clinical improvements noted in terms of the original degree of lameness.
Low-frequency US is a new and novel technique that does not require sedation of horses during treatment. It is also a very easy technique to use and can not only be performed in a routine Veterinary Clinic or field setting but is very well tolerated by horses. As a technique, it is known to produce cavitation [21]. Indeed, the use of cavitation for histotripsy is well known, particularly as a means of treating prostate diseases, liver cancer, kidney stones, and thrombolysis [21]. However, low-frequency US has been shown to have other effects besides those of tissue ablation. In a very recent article, Wu et al [22] were able to show that low-frequency US enhances and induces autophagy. These authors were able to document that low-frequency US induces cavitation in tissue, primarily affecting the endoplasmic reticulum and resulting in stress (referred to as endoplasmic reticulum stress [ERs]) [22]. ERs is known to caused impaired function of the endoplasmic reticulum, leading to accumulation of misfolded and unfolded proteins [23].Of particular interest is the observation that low-frequency-induced ERs significantly increase autophagy levels within targeted tissues [22].
Autophagy is a dynamic multistep process in which essential autophagy genes participate in forming double-membrane structures that engulf damaged cellular proteins, lipids, and organelles before delivering them to lysosomes for subsequent degradation [24-26]. Under normal physiological conditions, autophagic activity is low, but it can be induced in response to a variety of stimuli, including nutrient limitation, oxidative stress, hypoxia, increased metabolic demands, ERs, physiological agents, and inflammation [27]. In this way, autophagy contributes to cellular homeostasis by removing damaged organelles and maintaining their normal turnover [28].
Beside the direct activation of repair processes, cavitation bubbles also generate an effect called microstreaming [29]. The effects of therapeutic US in this way (diathermy, acoustical streaming, and microstreaming) are well known and documented [29,30]. Indeed it is known that different transducers can be used to induce specific conditions; for example, diathermy to revascularize chronic conditions and acoustical streaming and microstreaming to facilitate the drainage of edema [31-34].
Ligaments and tendons are frequently injured due to trauma and overuseevery often in young and physically active subjects [35,36]. This most commonly arises because of that rapid development of muscle tissue under training, giving rise to extremely rapid and high levels of force without being compensated by equally rapid changes in the ligaments and tendons [37,38]. Although adaptation of tendons has been demonstrated [39,40], this adaption is slower than the development of a strong muscle during periods of regular training exercise, with rapid increases in force production resulting in ligament and tendon damage at a structural level [41]. A number of systemic diseases are also known to be associated with general defects in matrix metabolism and structure that compromise ligament and tendon strength and elasticity, moreover the term “spontaneous tendon rupture” is being used on a more regular basis in connection with sport [42].
A few animal studies have been performed using an overuse protocol [43-45], where rats ran with a velocity of 17 m/min, 5 d/wk, 1 hr/d, either uphill or downhill for a period of between 2 and 16 weeks. In such experiments, a decreased collagen fiber organization and increased number of cell nuclei was observed [46,47]. Yet, exactly how the connective tissue of the ligaments and tendons manages the active tension transfer from bone to bone or muscle to bone, respectively, and whether this tension transfer is affected by inflammation, remains unclear.